FDA Bioequivalence: What It Means for Generic Drugs and Your Health
When you pick up a generic pill, you want to know it’ll do the same job as the brand-name version. That’s where FDA bioequivalence, a scientific standard used by the U.S. Food and Drug Administration to prove generic drugs perform the same way in the body as their brand-name counterparts. Also known as therapeutic equivalence, it’s the quiet rule that keeps millions of Americans safe while saving billions every year. Without it, generics could be weaker, slower, or even unsafe—but the FDA doesn’t allow that. Every generic drug must pass strict bioequivalence tests before it hits pharmacy shelves.
These tests measure how fast and how much of the drug enters your bloodstream. If the generic matches the brand within a narrow range—usually 80% to 125% of the original—it’s approved. This isn’t guesswork. It’s lab-tested, data-driven, and required by law. You’ll find this process mentioned in posts about generic substitution, the practice of replacing brand-name drugs with FDA-approved equivalents, often required in workers’ compensation and employer health plans, and in discussions about pharmacy dispensing errors, mistakes that can happen when pharmacists misidentify or mislabel drugs that should be bioequivalent. The system works because it’s built on hard numbers, not opinions.
But bioequivalence isn’t just about chemistry. It’s about trust. When your insurance pushes you toward a cheaper pill, you need to know it won’t cause unexpected side effects or fail to control your condition. That’s why posts on medication safety, the practice of ensuring drugs work as intended without harmful interactions or errors always circle back to this standard. Even when drugs interact—like phenytoin and warfarin, or calcium and thyroid meds—their bioequivalence ensures the baseline is reliable. If the generic doesn’t deliver the same amount of active ingredient, everything else falls apart.
Some people worry that generics are ‘inferior.’ But the FDA doesn’t approve a drug just because it’s cheap. It approves it because it’s proven to work the same. And when you see posts about counterfeit drugs, fake medications that bypass all testing and safety checks, you’ll understand why bioequivalence matters. Real generics are tested. Fakes aren’t. The difference is life or death.
You’ll find real-world examples in the articles below—from how formularies use bioequivalence to cut costs, to how pharmacists verify packaging to avoid mix-ups, to how patients handle switching medications without losing control of their condition. This isn’t theory. It’s daily practice. And if you’re taking generics, you’re already relying on it. The question isn’t whether bioequivalence works—it’s whether you know how to use that knowledge to protect yourself.

In Vivo vs In Vitro Bioequivalence Testing: When Each Is Used
In vivo and in vitro bioequivalence testing are two ways to prove generic drugs work like brand-name versions. In vivo uses human subjects; in vitro uses lab tests. Learn when each is required and why regulators are shifting toward lab-based methods.
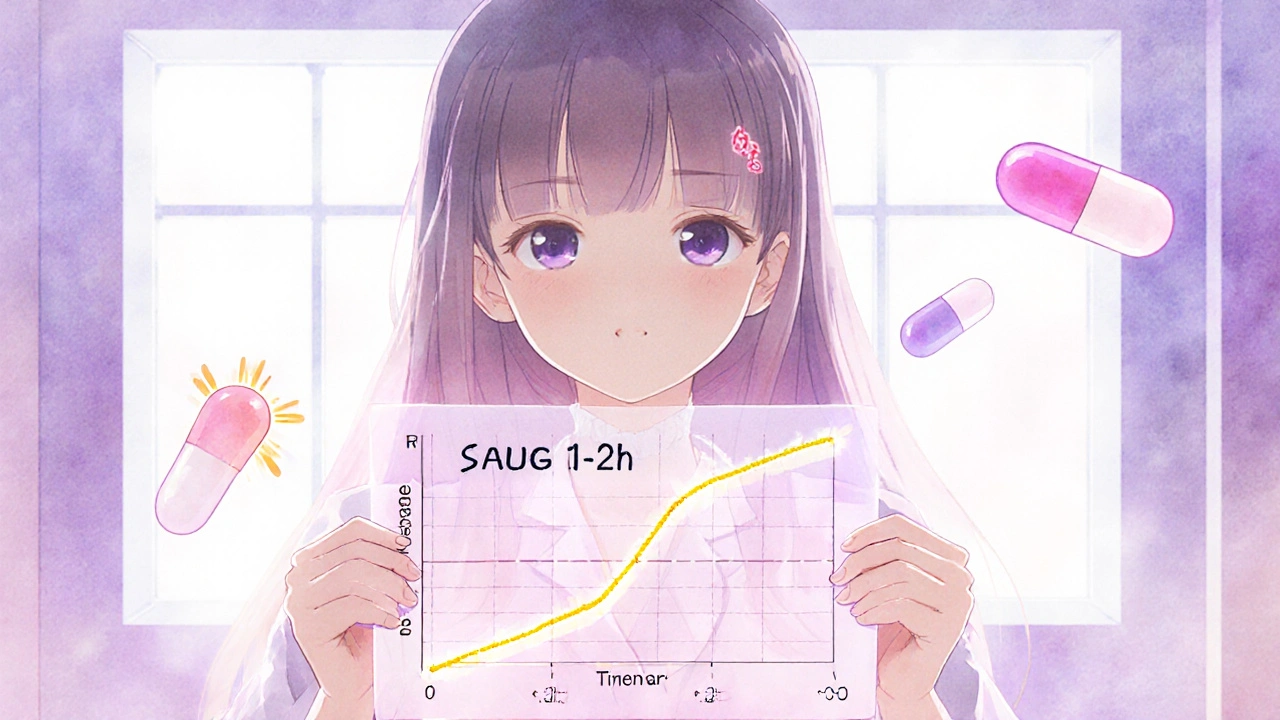
Partial AUC in Bioequivalence: How Advanced Metrics Ensure Drug Safety and Effectiveness
Partial AUC is a precise pharmacokinetic tool used to ensure generic drugs match brand-name versions in how quickly they're absorbed. It's now required for complex formulations like extended-release opioids and CNS drugs.
